How To Use Electronegativity To Find Polarity
Electronegativity polar covalent bonds compounds differences explain Electronegativity polarity values relative ib chemistry bonds Electronegativity and polarity
4.3: Molecular Shape and Molecular Polarity - Chemistry LibreTexts
Polar bond covalent bonds chemistry nonpolar polarity molecular shape properties electronegativity general bonding ionic electron vs molecules lewis principles chemical Electronegativity and bond polarity Electronegativity polarity h2o bonds periodic atoms bonding socratic electron covalent
Electronegativity polarity f321 ws simple kb ppt resources
Solved based on electronegativity values, in which of theElectronegativity based Polarity electronegativity bond chemistryElectronegativity differences explain polar bonds in covalent compounds.
Why is h2o a polar bond?4.3: molecular shape and molecular polarity Ch104: chapter 7 – solutions – chemistryWhat kinds of molecules are polar? + example.

Electronegativity and bond polarity
Compounds covalent bonds electronegativity nonpolar molecular atom ionic predict ch150 molecule ch104 characteristics question ch105 represents electron solubility substance wou4.2 relative polarity of bonds from electronegativity values [sl ib Polarity electronegativityMolecule polarity molecules bonds socratic strongest hydrogen bonding dipole electrons kinds illustrates versus.
How to tell if a bond is polarElectronegativity polarity bond 4.2b-electronegativity and polarityElectronegativity polarity tumblr.

Electronegativity polar bond values chemical tell if formula do slide use
.
.


Solved Based on electronegativity values, in which of the | Chegg.com

What kinds of molecules are polar? + Example

Why is H2O a polar bond? | Socratic

4.3: Molecular Shape and Molecular Polarity - Chemistry LibreTexts

Electronegativity differences explain Polar bonds in covalent compounds

Electronegativity and Polarity - cheMYSTERY

Electronegativity and Bond Polarity - Revision for A-level Chemistry

CH104: Chapter 7 – Solutions – Chemistry
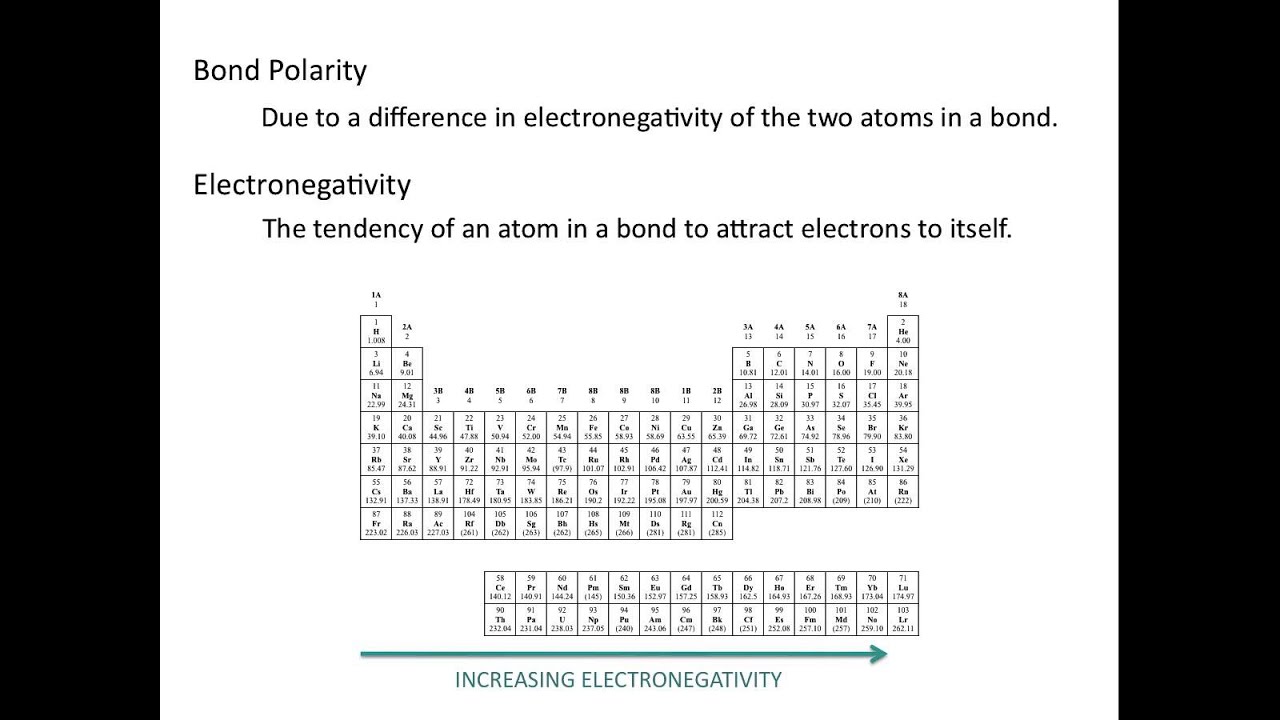
Electronegativity and Bond Polarity - Chemistry Tutorial - YouTube